Terms and Conditions for data source contributors and users of the EMIF Catalogue
About EMIF
EMIF, the European Medical Information Framework, is a joint multidisciplinary research project that is building an efficient integrated framework for the re-use of health and life sciences data to support novel research. The EMIF Platform will enable verified research users to securely analyse multiple, diverse data sources through a single portal, and thereby enable data users and data sources to collaborate throughout the research lifecycle from data discovery to data access and data analysis.
The ultimate aim of EMIF is to support maximum scientific research value to be derived from health data whilst ensuring patient privacy, and safeguarding the positive reputation and continuity of longitudinal research, and of research organisations.
The EMIF Platform will eventually offer a range of information, data provisioning and analytic services which are intended to support new research from health data. This Code of Practice only relates to the EMIF Catalogue currently.
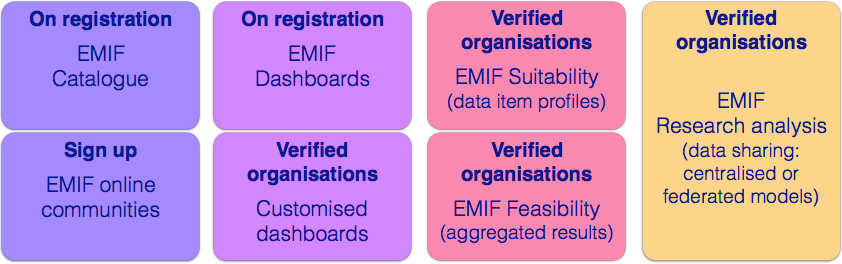
The EMIF Catalogue
The EMIF Catalogue
provides a list of all the data sources registered by EMIF, through a portal and search tools, to enable potential data users to discover data sources that are most relevant to their research needs, according to a variety of data source and dataset descriptors.
Through the Catalogue, EMIF provides registered users with descriptive information about the data sources that are offering access to their research data via the EMIF Platform, including lists of publications showing how the data from that data source has already contributed to research.
Potential research users of EMIF may discover, through the EMIF Catalogue, what data are accessible for sharing and any constraints on their use, how requests are to be made and how data sharing requests will be assessed.
Code of practice for data sources contributing to the Catalogue
Data sources shall provide accurate descriptive information about (i) their organisation, and (ii) its data subjects and data holdings (in a fully anonymized way), using the standard descriptors and file formats specified by EMIF.
Data sources shall declare and maintain, through the Catalogue, information about what their original source health data is:
- in the public domain without restriction;
- available to bona fide researchers for shared use within the terms of any applicable participant consent and ethical approval, and is not restricted by Intellectual Property Rights;
- described within the Catalogue, but is not available for analysis level access because of Intellectual Property Rights (IPR) or the limited scope of historically-obtained participants’ consent, and so cannot be shared with external researchers.
Progress with any current wave(s) of collection, and the expected availability of that data, should be updated regularly, at least annually.
A list of variables or categories of variables that are browsable and searchable should be provided via the EMIF Catalogue, and regularly updated or confirmed, at least annually. This variable list should ideally include metadata (value lists, completion rates, data quality indicators etc.).
Data sources shall ensure that an up-to-date, informative description of the study or studies that have contributed research data to the data source is readily discoverable via the EMIF Catalogue, and is maintained.
Data sources shall ensure that the contact details they provide will be updated when needed.
Data sources represent and warrant to University of Aveiro that (i) the information they provide for inclusion in the EMIF Catalogue is correct; (ii) that they have all the rights to contribute such information to the EMIF Catalogue; and (iii) that this information can be included in the EMIF Catalogue and made available to the users.
Code of practice for University of Aveiro relating to the Catalogue
University of Aveiro will:
- undertake reasonable efforts to provide data sources with a secure means that enables them to provide and maintain Catalogue entries, and protect these against tampering by other parties.
- undertake reasonable efforts to support data sources to enable updates and corrections to be made in a timely fashion, and ensure that any corrections of error or rectifications to prevent breaches of privacy are made.
- undertake reasonable efforts to verify the identity of users registering to access its Catalogue and tools.
- undertake reasonable efforts to ensure that all data provided by a data source is acknowledged and attributed within its portal and tools.
Code of practice for users of the EMIF Catalogue and Dashboards
Any publicly accessible materials, such as publications or reports or web pages that contain information derived from the EMIF Catalogue must acknowledge EMIF, and the original data sources where applicable. Citation text is provided within the Catalogue application.
EMIF users may not reproduce portions of the Catalogue in any external publication or web page or other form that serves to duplicate the purposes of the EMIF Catalogue.
In accessing the EMIF Catalogue, users must at all times comply with all applicable laws and regulations as well as ethical requirements.
More specific Terms & Conditions may be defined by each Catalogue community to regulate internal users’ membership and data access rules